Titration Neutralization Equation . the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In addition, we investigate titration curves that are plots. solving titration problems problem: This titrant addition involves a stoichiometric amount of base (the equivalence point), and so only. learn how to calculate the concentration or volume of a solution using titration, a chemical technique to measure the. this chapter explores the principles of acid/base titrations. (c) titrant volume = 25.00 ml. What is the molarity of a solution of hcl if 20.00 ml of this solution requires 17.56.
from www.vrogue.co
learn how to calculate the concentration or volume of a solution using titration, a chemical technique to measure the. What is the molarity of a solution of hcl if 20.00 ml of this solution requires 17.56. (c) titrant volume = 25.00 ml. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. This titrant addition involves a stoichiometric amount of base (the equivalence point), and so only. solving titration problems problem: this chapter explores the principles of acid/base titrations. In addition, we investigate titration curves that are plots.
Ph Indicators Titration Curves Teaching Resources vrogue.co
Titration Neutralization Equation solving titration problems problem: In addition, we investigate titration curves that are plots. this chapter explores the principles of acid/base titrations. solving titration problems problem: learn how to calculate the concentration or volume of a solution using titration, a chemical technique to measure the. This titrant addition involves a stoichiometric amount of base (the equivalence point), and so only. What is the molarity of a solution of hcl if 20.00 ml of this solution requires 17.56. (c) titrant volume = 25.00 ml. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base.
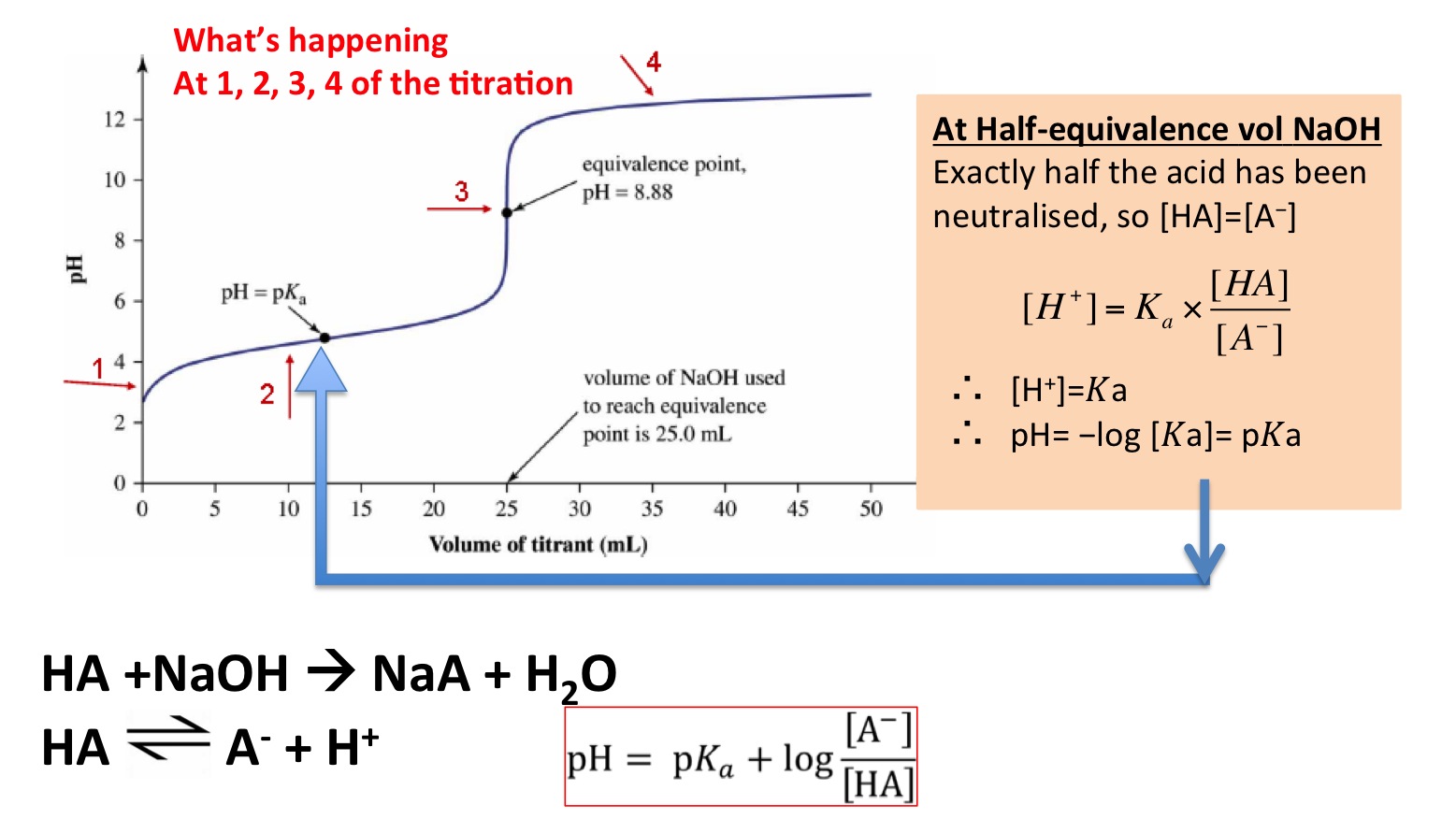
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Titration Neutralization Equation this chapter explores the principles of acid/base titrations. In addition, we investigate titration curves that are plots. (c) titrant volume = 25.00 ml. solving titration problems problem: the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. What is the molarity of a solution of hcl. Titration Neutralization Equation.
From www.inf-inet.com
Neutralisation Berechnen Titration Neutralization Equation this chapter explores the principles of acid/base titrations. learn how to calculate the concentration or volume of a solution using titration, a chemical technique to measure the. This titrant addition involves a stoichiometric amount of base (the equivalence point), and so only. (c) titrant volume = 25.00 ml. the above equation works only for neutralizations in. Titration Neutralization Equation.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Neutralization Equation this chapter explores the principles of acid/base titrations. (c) titrant volume = 25.00 ml. What is the molarity of a solution of hcl if 20.00 ml of this solution requires 17.56. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. solving titration problems problem: In. Titration Neutralization Equation.
From www.slideserve.com
PPT Neutralization & Titration PowerPoint Presentation, free download Titration Neutralization Equation solving titration problems problem: In addition, we investigate titration curves that are plots. learn how to calculate the concentration or volume of a solution using titration, a chemical technique to measure the. (c) titrant volume = 25.00 ml. What is the molarity of a solution of hcl if 20.00 ml of this solution requires 17.56. this. Titration Neutralization Equation.
From www.youtube.com
Solving AcidBase Titration Problems YouTube Titration Neutralization Equation this chapter explores the principles of acid/base titrations. What is the molarity of a solution of hcl if 20.00 ml of this solution requires 17.56. learn how to calculate the concentration or volume of a solution using titration, a chemical technique to measure the. (c) titrant volume = 25.00 ml. In addition, we investigate titration curves that. Titration Neutralization Equation.
From saylordotorg.github.io
AcidBase Titrations Titration Neutralization Equation In addition, we investigate titration curves that are plots. This titrant addition involves a stoichiometric amount of base (the equivalence point), and so only. learn how to calculate the concentration or volume of a solution using titration, a chemical technique to measure the. What is the molarity of a solution of hcl if 20.00 ml of this solution requires. Titration Neutralization Equation.
From printablelistmcguire.z22.web.core.windows.net
Chemical Reaction In Acidbase Neutralization Titration Neutralization Equation (c) titrant volume = 25.00 ml. learn how to calculate the concentration or volume of a solution using titration, a chemical technique to measure the. What is the molarity of a solution of hcl if 20.00 ml of this solution requires 17.56. this chapter explores the principles of acid/base titrations. In addition, we investigate titration curves that. Titration Neutralization Equation.
From exoqbgfse.blob.core.windows.net
Sample Lab Report On Acid Base Titration at Michelle Hamilton blog Titration Neutralization Equation learn how to calculate the concentration or volume of a solution using titration, a chemical technique to measure the. What is the molarity of a solution of hcl if 20.00 ml of this solution requires 17.56. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. solving. Titration Neutralization Equation.
From byjus.com
Write the neutralization reaction between Hydrochloric acid HCI and Titration Neutralization Equation the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. learn how to calculate the concentration or volume of a solution using titration, a chemical technique to measure the. This titrant addition involves a stoichiometric amount of base (the equivalence point), and so only. What is the molarity. Titration Neutralization Equation.
From dxofwhltu.blob.core.windows.net
Titration Chemistry Ia Ideas at Selma Schoenrock blog Titration Neutralization Equation learn how to calculate the concentration or volume of a solution using titration, a chemical technique to measure the. This titrant addition involves a stoichiometric amount of base (the equivalence point), and so only. this chapter explores the principles of acid/base titrations. the above equation works only for neutralizations in which there is a 1:1 ratio between. Titration Neutralization Equation.
From www.slideserve.com
PPT Neutralization & Titration PowerPoint Presentation, free download Titration Neutralization Equation the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In addition, we investigate titration curves that are plots. What is the molarity of a solution of hcl if 20.00 ml of this solution requires 17.56. this chapter explores the principles of acid/base titrations. solving titration problems. Titration Neutralization Equation.
From www.slideserve.com
PPT Neutralization reaction PowerPoint Presentation, free download Titration Neutralization Equation the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. solving titration problems problem: (c) titrant volume = 25.00 ml. This titrant addition involves a stoichiometric amount of base (the equivalence point), and so only. What is the molarity of a solution of hcl if 20.00 ml. Titration Neutralization Equation.
From www.ck12.org
Neutralization Reaction CK12 Foundation Titration Neutralization Equation This titrant addition involves a stoichiometric amount of base (the equivalence point), and so only. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. (c) titrant volume = 25.00 ml. What is the molarity of a solution of hcl if 20.00 ml of this solution requires 17.56.. Titration Neutralization Equation.
From chem4three.blogspot.ca
CHEMISTRY 11 TITRATIONS Titration Neutralization Equation the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. learn how to calculate the concentration or volume of a solution using titration, a chemical technique to measure the. this chapter explores the principles of acid/base titrations. solving titration problems problem: (c) titrant volume =. Titration Neutralization Equation.
From lessonmagicgast.z22.web.core.windows.net
The Equation Shows A Neutralization Reaction Titration Neutralization Equation this chapter explores the principles of acid/base titrations. This titrant addition involves a stoichiometric amount of base (the equivalence point), and so only. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In addition, we investigate titration curves that are plots. solving titration problems problem: What. Titration Neutralization Equation.
From www.slideserve.com
PPT Neutralization Reactions PowerPoint Presentation, free download Titration Neutralization Equation learn how to calculate the concentration or volume of a solution using titration, a chemical technique to measure the. this chapter explores the principles of acid/base titrations. solving titration problems problem: (c) titrant volume = 25.00 ml. This titrant addition involves a stoichiometric amount of base (the equivalence point), and so only. In addition, we investigate. Titration Neutralization Equation.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Titration Neutralization Equation learn how to calculate the concentration or volume of a solution using titration, a chemical technique to measure the. What is the molarity of a solution of hcl if 20.00 ml of this solution requires 17.56. (c) titrant volume = 25.00 ml. the above equation works only for neutralizations in which there is a 1:1 ratio between. Titration Neutralization Equation.
From www.ck12.org
Neutralization Reaction and Net Ionic Equations for Neutralization Titration Neutralization Equation What is the molarity of a solution of hcl if 20.00 ml of this solution requires 17.56. This titrant addition involves a stoichiometric amount of base (the equivalence point), and so only. this chapter explores the principles of acid/base titrations. learn how to calculate the concentration or volume of a solution using titration, a chemical technique to measure. Titration Neutralization Equation.